


Allecra Therapeutics, founded in 2013, is a private, clinical-stage
biopharmaceutical company developing novel therapies to combat
antibiotic resistance by overcoming emergent resistance mechanisms.
Lead product candidate, cefepime/enmetazobactam, has shown
superiority over standard of care in patients with complicated
urinary tract infections (cUTIs) in a randomized, controlled Phase 3
trial, and the Company is preparing submissions for marketing
approval in the U.S. and EU based on these results. The Company has
significant patent protection covering proprietary enmetazobactam in
major territories.
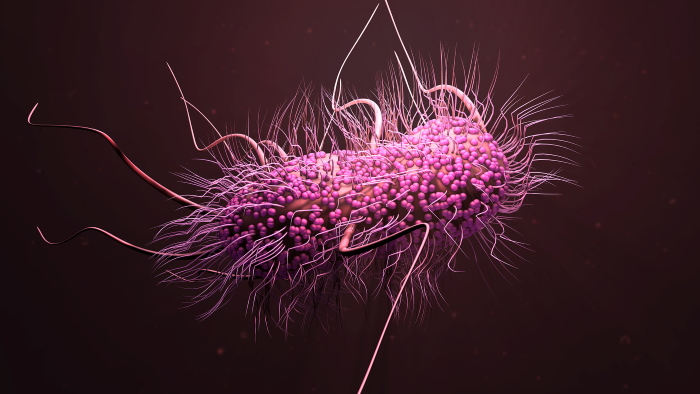

β-lactams were once the most widely used antibiotic class because of
their known safety and efficacy. Unfortunately, the extensive use of
these antibiotics resulted in the emergence of Extended-Spectrum
β-lactam resistant pathogens (ESBLs). Today, ESBLs represent a
worldwide problem, with high and growing prevalence in both
developed and developing countries.
What can combat ESBLs?
Antibiotics that combine a β-lactams with a β-lactamase inhibitor,
such as the widely used piperacillin-tazobactam, or another class of
antibiotics called carbapenems. Unfortunately,
piperacillin/tazobactam is now also suffering from a growing
ESBL-mediated resistance and carbapenem use is restricted by
hospitals to prevent the growth of additional resistant pathogens.
Cefepime/enmetazobactam has successfully completed a pivotal Phase
III trial (ALLIUM) in complicated urinary tract infections (cUTI).
The ALLIUM trial was a multi-center, randomized, controlled,
double-blind, global study that enrolled 1,034 patients. Patients
received either cefepime/enmetazobactam (FPE) or
piperacillin-tazobactam (PTZ) via intravenous infusion. The study
involved 112 sites in 19 countries. The trial demonstrated
superiority in its primary endpoint, with FPE showing a significant
improvement over PTZ in the composite success outcome of clinical
cure and microbiological eradication at the test-of-cure visit. In
the trial, FPE was well tolerated, with 4.3% of patients reporting
serious adverse events vs. 3.7 % with PTZ (0.2% vs. 0.6% assessed as
drug related), suggesting a comparable safety profile to PTZ.
Based on the positive results from this study, Allecra is preparing
to submit applications for marketing approval in both the U.S. and
Europe, which are expected to be completed in the second half of
2021.


Allecra is actively seeking partnerships for the further development
and commercialization of cefepime/enmetazobactam. We are open to
various opportunities, including global as well as regional licensing
agreements.
Business development contact:
Allecra Therapeutics GmbH
Andreas Kranzusch
Chief Financial Officer
ir@allecra.com
+49 761 45899872
For more information, please contact:
Allecra Therapeutics GmbH
Andreas Kranzusch
Chief Financial Officer
This email address is being protected from spambots. You need JavaScript enabled to view it.
+49 761 45899872
Trophic Communication
Gretchen Schweitzer
Tel: +49-89-23887735
This email address is being protected from spambots. You need JavaScript enabled to view it.
Notes on data protection
We would like to thank you for visiting our homepage www.allecra.com and are very pleased about your interest in our company. Data protection is of a particularly high priority for the management of Allecra Therapeutics GmbH. In principle, the use of our website is possible without any indication of personal data. However, if a data subject wants to use special services of our enterprise via our website, processing of personal data could become necessary. If processing of personal data is necessary and there is no other legal basis for such processing, we will generally obtain the consent of the data subject.
The processing of personal data, such as the name, address, e-mail address, or telephone number of a data subject shall always be in line with the German Data Protection Regulation (Datenschutz-Grundverordnung - DSGVO), and in accordance with the country-specific data protection regulations applicable to the Allecra Therapeutics GmbH (e.g. Federal Data Protection Act, Telemedia Act). By means of this data protection notice, we would like to inform you about the type, scope and purpose of the personal data collected, used and processed by us. Furthermore, data subjects are informed of their rights by means of this data protection notice.
As the controller, the Allecra Therapeutics GmbH has implemented numerous technical and organizational measures to ensure the most complete protection of personal data processed through this website. Nevertheless, Internet-based data transmissions can always be vulnerable to security risks, so that absolute protection cannot be guaranteed. For this reason, every data subject is free to transmit personal data to us by alternative means, for example by telephone.
All terms used in these notes are non-gender specific.
The data protection information of Allecra Therapeutics GmbH is based on the terms used by the European Directive and Ordinance when issuing the General Data Protection Regulation (GDPR). Our data protection information should be easy to read and understand for the public as well as for our customers and business partners. To ensure this, we would like to explain the terms used in advance.
We use the following terms, among others, in this Privacy Notice:
Personal data is any information relating to an identified or identifiable natural person (hereinafter "data subject"). An identifiable natural person is one who can be identified, directly or indirectly, in particular by reference to an identifier such as a name, an identification number, location data, an online identifier or to one or more factors specific to the physical, physiological, genetic, mental, economic, cultural or social identity of that natural person.
Data subject is any identified or identifiable natural person whose personal data are processed by the controller.
Processing means any operation or set of operations which is performed upon personal data, whether or not by automatic means, such as collection, recording, organization, filing, storage, adaptation or alteration, retrieval, consultation, use, disclosure by transmission, dissemination or otherwise making available, alignment or combination, restriction, erasure or destruction.
Restriction of processing is the marking of stored personal data with the aim of limiting their future processing.
Profiling is any type of automated processing of personal data that consists of using such personal data to evaluate certain personal aspects relating to a natural person, in particular to analyze or predict aspects relating to that natural person's job performance, economic situation, health, personal preferences, interests, reliability, behavior, location or change of location.
Pseudonymization is the processing of personal data in such a way that the personal data can no longer be attributed to a specific data subject without the use of additional information, provided that such additional information is kept separate and is subject to technical and organizational measures to ensure that the personal data is not attributed to an identified or identifiable natural person.
The controller or controller is the natural or legal person, public authority, agency or other body which alone or jointly with others determines the purposes and means of the processing of personal data. Where the purposes and means of such processing are determined by Union or Member State law, the controller or the specific criteria for its designation may be provided for under Union or Member State law.
Processor means a natural or legal person, public authority, agency or other body that processes personal data on behalf of the Controller.
A recipient is a natural or legal person, public authority, agency or other body to whom personal data are disclosed, whether or not a third party. However, public authorities that may receive personal data in the context of a specific investigative task under Union or Member State law shall not be considered as recipients.
Third party means a natural or legal person, public authority, agency or other body other than the data subject, the controller, the processor and the persons authorized to process the personal data under the direct responsibility of the controller or the processor.
Consent shall mean any freely given indication of the data subject's wishes for the specific case in an informed and unambiguous manner in the form of a statement or any other unambiguous affirmative act by which the data subject indicates that he or she consents to the processing of personal data relating to him or her.
The responsible party within the meaning of the General Data Protection Regulation, other data protection laws applicable in the Member States of the European Union and other provisions of a data protection nature is:
Allecra Therapeutics GmbH
Wallbrunnstrasse 24
79539 Lörrach
Germany
Tel: +49-761-45899872
Email: This email address is being protected from spambots. You need JavaScript enabled to view it. Internet: www.allecra.com
The controller has appointed a data protection officer; he can be reached as follows:
Viehoff Consult e.K. Stephan Viehoff
Senior Consultant & Owner
Im Hahn 28
52224 Stolberg
Tel.: +49-2402-99 83 83 - 0
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Any data subject may contact our data protection officer directly at any time with any questions or suggestions regarding data protection.
On the basis of Art. 6 para 1 lit. f DSGVO, our website collects a series of general data and information with each call by a data subject or an automated system. This general data and information is temporarily stored in the log files of the server. The following can be recorded
When using these general data and information, the Allecra Therapeutics GmbH does not draw any conclusions about the data subject. This information is rather required in order to
This anonymously collected data and information is therefore evaluated statistically, on the one hand, and on the other hand, with the aim of increasing the data protection and data security of our company, in order to ultimately ensure an optimal level of protection for the personal data we process. The anonymous data of the server log files are stored separately from any personal data provided by a data subject.
In order to provide our online offer securely and efficiently, we use the services of one or more web hosting providers from whose servers (or servers managed by them) the online offer can be accessed. For these purposes, we may use infrastructure and platform services, computing capacity, storage space and database services, as well as security services and technical maintenance services.
The data processed as part of the provision of the hosting offer may include all information relating to the users of our online offer, which is generated as part of the use and communication. This regularly includes the IP address, which is necessary to be able to
deliver the contents of online offers to browsers, and all entries made within our online offer or from websites.
E-mail dispatch and hosting: The web hosting services we use also include the dispatch, receipt and storage of e-mails. For these purposes, the addresses of the recipients and senders as well as further information regarding the e-mail dispatch (e.g. the providers involved) and the contents of the respective e-mails are processed. The aforementioned data may also be processed for SPAM detection purposes. Please note that e-mails are generally not sent encrypted on the Internet. As a rule, e-mails are encrypted in transit, but (unless a so-called end-to-end encryption method is used) not on the servers from which they are sent and received. We can therefore not assume any responsibility for the transmission path of the e-mails between the sender and the reception on our server.
Services used and service providers:
Zendesk:
Service provider: Zendesk, Inc, 1019 Market Street in San Francisco, CA 94103, USA.; Website:
www.zendesk.deDatenschutzerklärung: https://www.zendesk.de/company/customers- partners/privacy-policy/.
We take appropriate technical and organizational measures in accordance with the legal requirements, taking into account the state of the art, implementation costs, the nature, scope, circumstances and purposes of the processing, as well as the different probabilities of occurrence and the extent of the threat to the rights and freedoms of natural persons, in order to ensure a level of protection appropriate to the risk.
The measures include, in particular, safeguarding the confidentiality, integrity and availability of data by controlling physical and electronic access to the data as well as the access, input, transfer, safeguarding of availability and its separation. Furthermore, we have established procedures to ensure the exercise of data subjects' rights, the deletion of data, and responses to data compromise. Furthermore, we take the protection of personal data into account as early as the development or selection of hardware, software and processes in accordance with the principle of data protection through technology design and data protection-friendly default settings.
If it is possible for us or if it is not necessary to store the IP address, we will shorten your IP address or have it shortened. In the case of IP address shortening, also known as "IP masking", the last octet, i.e. the last two numbers of an IP address, is deleted (in this context, the IP address is an identifier individually assigned to an Internet connection by the online access provider). The purpose of shortening the IP address is to prevent or make it significantly more difficult to identify a person by their IP address.
To protect your data transmitted by visiting our website, we use TLS or SSL encryption. You can recognize such encrypted connections by the prefix "https://" or the lock in the address bar of your browser.
In the course of our processing of personal data, it may happen that the data is transferred to other bodies, companies, legally independent organizational units or persons or that it is disclosed to them. Recipients of this data may include, for example, payment institutions in the context of payment transactions, service providers commissioned with IT tasks or providers of services and content that are integrated into a website. In such a case, we observe the legal requirements and conclude appropriate contracts or agreements with the recipients of your data in particular, which serve to protect your data.
If we process data in a third country (i.e. outside the European Union (EU), the European Economic Area (EEA)) or the processing takes place in the context of the use of third-party services or the disclosure or transfer of data to other persons, bodies or companies, this is only done in accordance with the legal requirements.
Subject to express consent or contractually or legally required transfer, we only process or have the data processed in third countries with a recognized level of data protection or on the basis of special guarantees, such as contractual obligation through so-called standard protection clauses of the EU Commission, the existence of certifications or binding internal data protection regulations (Art. 44 to 49 DSGVO). More information on this can be found on an information page of the EU Commission at https://ec.europa.eu/info/law/law-topic/data-protection/international-dimension-data-protection_de.
Among other things, we have also integrated tools from companies based in the USA on our website. If these tools are active, your personal data may be transferred to the US servers of the respective companies.
For a long time, the basis for the data transfer was an adequacy decision of the EU Commission based on the EU-US Privacy Shield agreement, to which many American companies have committed. However, in its ruling of July 16, 2020, the ECJ declared this agreement invalid (C-311/18).
We would like to point out that the USA is not a safe third country in the sense of EU data protection law. US companies are obliged to hand over personal data to security authorities without you as the data subject being able to take legal action against this. It can therefore not be ruled out that US authorities (e.g. intelligence services) process, evaluate and permanently store your data located on US servers for monitoring purposes. We have no influence on these processing activities.
If we use the tools with your explicit consent, you have the option at any time to revoke your consent to the processing of your personal data with effect for the future via our consent tool. You will find further information on this in the respective chapters.
We use cookies on our website. Cookies are small text files that are stored by us on your computer system via your Internet browser (e.g. Mozilla Firefox, Microsoft Explorer) when you visit our website and may be stored there for only one session or for a longer period ("persistent").
Numerous websites and servers use cookies. Many cookies contain a so-called cookie ID. A cookie ID is a unique identifier of the cookie. It consists of a string of characters by which Internet pages and servers can be assigned to the specific Internet browser in which the cookie was stored. This enables the visited Internet pages and servers to distinguish the individual Internet browser of the data subject from other Internet browsers that contain other cookies. A specific internet browser can be recognized and identified via the unique cookie ID.
Through the use of cookies, we can provide the users of our website with more user-friendly services that would not be possible without the cookie setting. We only use technically necessary cookies, for which neither your consent nor a cookie consent management is required.
Most Internet browsers are preset to accept cookies by default. However, you can configure your respective Internet browser so that it only accepts certain cookies or no cookies at all. However, we would like to point out that you may then no longer be able to use functions of our website and may instead receive warning or error messages if cookies are disabled by your browser settings on our website.
In your browser settings, you can also delete cookies already stored in your Internet browser. Furthermore, it is possible to set your internet browser to notify you before cookies are stored. Since the various Internet browsers may differ in their respective modes of operation, we ask you to refer to the respective help menu of your Internet browser for configuration options. Notes on the most common Internet browsers can be found here:
If you want a comprehensive overview of all third-party accesses to your Internet browser, we recommend installing specially developed plugins for this purpose.
We recommend that you always log out completely after you stop using a terminal device that you share with other people and whose Internet browser is set to allow cookies.
You have the option of contacting us by mail, telephone, fax, e-mail or via the Internet (e.g. contact forms, social media).
If you contact us by mail, we may process in particular your address data (e.g. surname, first name, street, city, postal code), date and time of receipt of the mail as well as those data which result from your letter itself.
If contact is made, a secretarial service may also process your data and transfer it to us after you have contacted us. Depending on the data you enter here, we will then contact you by phone, fax or e-mail and call you back or write to you if necessary.
If you contact us by telephone, your telephone number in particular and, if necessary, your name, e-mail address, time of call and details of your request will be processed during the conversation upon request.
If you contact us by fax, in particular the fax number or the sender ID as well as the data resulting from the fax will be processed.
If you contact us by e-mail, we will process in particular your e-mail address, the time of the e-mail and the data resulting from the message text (including attachments, if applicable).
The purpose of processing the above-mentioned data is to process your contact request and to be able to contact you in order to respond to your request. The legal basis for the processing of personal data described here is contract performance and pre-contractual inquiries pursuant to Art. 6 (1) lit. b as well as our legitimate interest pursuant to Art. 6 para 1 lit. f DSGVO. Our legitimate interest is to offer you the possibility to contact us at any time and to be able to answer your inquiries.
The personal data will only be processed as long as it is necessary for the processing of the contact request.
We process personal data for the purpose of promotional communication, which may take place via various channels, such as e-mail, telephone, mail or fax. In this context, we observe the legal requirements and obtain the necessary consents, unless the communication is permitted by law.
Recipients have the right to revoke consent given at any time or to object to promotional communications at any time.
After revocation or objection, we may store the data required to prove consent for up to three years based on our legitimate interests before deleting it. The processing of this data is limited to the purpose of a possible defense against claims. An individual deletion request is possible at any time, provided that the former existence of consent is confirmed at the same time.
Based on statutory provisions, the website of Allecra Therapeutics GmbH contains data that enables a quick electronic contact to our company as well as direct communication with us,
which also includes a general address of the so-called electronic mail (e-mail address). If you contact us by e-mail or via a contact form, the personal data you provide will be stored automatically. Such personal data transmitted by you to us on a voluntary basis will be stored for the purpose of processing or contacting you. This personal data will not be passed on to third parties.
We process and store your personal data only for the period of time necessary to achieve the purpose of storage or if this has been provided for by the European Directive and Regulation Maker or another legislator in laws or regulations to which we are subject.
If the purpose of storage no longer applies or if a storage period prescribed by the European Directive and Regulation or another competent legislator expires, the personal data will be routinely blocked or deleted in accordance with the statutory provisions.
Every data subject shall have the right, granted by the European Directive and the Regulation, to obtain confirmation from the controller as to whether personal data concerning him or her are being processed. If a data subject wishes to exercise this right, he or she may, at any time, contact any employee of the controller.
Any person concerned by the processing of personal data has the right granted by the European Directive and Regulation to obtain at any time from the controller, free of charge, information about the personal data stored about him or her and a copy of that information. Furthermore, the European Directive and Regulation has granted the data subject access to the following information:
Furthermore, the data subject shall have the right to obtain information as to whether personal data have been transferred to a third country or to an international organization. If this is the case, the data subject also has the right to obtain information about the appropriate safeguards in connection with the transfer.
If a data subject wishes to exercise this right of access, he or she may, at any time, contact any employee of the controller.
Any person affected by the processing of personal data has the right granted by the European Directive and Regulation to request the immediate rectification of inaccurate personal data concerning him or her. Furthermore, the data subject has the right to request the completion of incomplete personal data - also by means of a supplementary declaration - taking into account the purposes of the processing.
If a data subject wishes to exercise this right to rectify, he or she may, at any time, contact any employee of the controller.
Any person concerned by the processing of personal data has the right, granted by the European Directive and Regulation, to obtain from the controller the erasure without delay of personal data concerning him or her, where one of the following reasons applies and insofar as the processing is not necessary:
If one of the aforementioned reasons applies, and a data subject wishes to arrange for the deletion of personal data stored by the Allecra Therapeutics GmbH, he or she may, at any time, contact any employee of the controller. The employee of Allecra Therapeutics GmbH will arrange for the erasure request to be complied with immediately.
If the personal data was made public by the Allecra Therapeutics GmbH and our company as the responsible party is obliged to delete the personal data pursuant to Art. 17 Para. 1 DSGVO, Allecra Therapeutics GmbH shall implement reasonable measures, including technical measures, to compensate other data controllers for processing the personal data published, taking into account the available technology and the cost of implementation, in order to inform the data subject that he or she has requested from those other data controllers to erase all links to the personal data or copies or replications of the personal data, unless the processing is necessary. The employee of the Allecra Therapeutics GmbH will arrange the necessary in individual cases.
Any person concerned by the processing of personal data has the right, granted by the European Directive and Regulation, to obtain from the controller the restriction of processing if one of the following conditions is met:
If one of the aforementioned conditions is met, and a data subject wishes to request the restriction of personal data stored by the Allecra Therapeutics GmbH, he or she may, at any time, contact any employee of the controller. The employee of the Allecra Therapeutics GmbH will arrange the restriction of the processing.
Any person concerned by the processing of personal data has the right, granted by the European Directive and Regulation, to receive the personal data concerning him or her, which have been provided by the data subject to a controller, in a structured, commonly used and machine-readable format. He or she also has the right to transmit this data to another controller without hindrance from the controller to whom the personal data have been provided, provided that the processing is based on consent pursuant to Article 6(1)(a) of the GDPR or Article 9(2)(a) of the GDPR or on a contract pursuant to Article 6(1)(b) of the GDPR and the processing is carried out by automated means, unless the processing is necessary for the performance of a task carried out in the public interest or in the exercise of official authority vested in the controller.
Furthermore, when exercising his or her right to data portability pursuant to Article 20(1) of the GDPR, the data subject has the right to obtain that the personal data be transferred directly from one controller to another controller, to the extent that this is technically feasible and provided that this does not adversely affect the rights and freedoms of other individuals.
In order to assert the right to data portability, the data subject may at any time contact any employee of the Allecra Therapeutics GmbH.
Any person affected by the processing of personal data has the right granted by the European Directive and Regulation to object at any time, on grounds relating to his or her particular situation, to the processing of personal data concerning him or her carried out on the basis of Article 6(1)(e) or (f) DSGVO. This also applies to profiling based on these provisions.
The Allecra Therapeutics GmbH shall no longer process the personal data in the event of the objection, unless we can demonstrate compelling legitimate grounds for the processing which override the interests, rights and freedoms of the data subject, or for the assertion, exercise or defence of legal claims.
If the Allecra Therapeutics GmbH processes personal data for direct marketing purposes, the data subject shall have the right to object at any time to processing of personal data for such marketing. This also applies to profiling, insofar as it is related to such direct marketing. If the data subject objects to Allecra Therapeutics GmbH to the processing for direct marketing purposes, Allecra Therapeutics GmbH will no longer process the personal data for these purposes.
In addition, the data subject has the right, on grounds relating to his or her particular situation, to object to processing of personal data concerning him or her which is carried out by the Allecra Therapeutics GmbH for scientific or historical research purposes, or for statistical purposes pursuant to Article 89(1) of the Data Protection Regulation, unless such processing is necessary for the performance of a task carried out in the public interest.
In order to exercise the right to object, the data subject may directly contact any employee of the Allecra Therapeutics GmbH or another employee. The data subject is also free to exercise his/her right to object in connection with the use of information society services, notwithstanding Directive 2002/58/EC, by means of automated procedures using technical specifications.
Any person concerned by the processing of personal data shall have the right, granted by the European Directive and the Regulation, not to be subject to a decision based solely on automated processing, including profiling, which produces legal effects concerning him or her or similarly significantly affects him or her, where such decision
Is the decision
Allecra Therapeutics GmbH shall take appropriate measures to safeguard the rights and freedoms as well as the legitimate interests of the data subject, which include at least the right to obtain the intervention of a data subject on the part of the responsible person, to express his or her point of view and to contest the decision.
If the data subject wishes to exercise the rights concerning automated decisions, he or she may, at any time, contact any employee of the controller.
Any person affected by the processing of personal data has the right granted by the European Directive and Regulation to withdraw consent to the processing of personal data at any time.
If the data subject wishes to exercise the right to withdraw the consent, he or she may, at any time, contact any employee of the controller.
Without prejudice to any other administrative or judicial remedy, you have the right to lodge a complaint with a supervisory authority, in particular in the Member State of your residence, workplace or the place of the alleged infringement, if you consider that the processing of personal data concerning you infringes the GDPR.
The supervisory authority to which the complaint has been lodged shall inform the complainant of the status and outcome of the complaint, including the possibility of a judicial remedy under Article 78 GDPR.
Contact details of the competent supervisory authority:
The State Commissioner for Data Protection and Freedom of Information
Königstrasse 10 a
70173 Stuttgart
Postal address:
P.O. Box 10 29 32
70025 Stuttgart
Tel.: 0711/615541-0
FAX: 0711/615541-15
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
We collect and process the personal data of applicants for the purpose of handling the application process. The processing may also take place electronically. This is particularly the case if an applicant sends us the relevant application documents electronically, for example by e-mail or via a web form on the website.
If we conclude an employment contract with an applicant, the data transmitted will be stored for the purpose of processing the employment relationship in compliance with the statutory provisions. If we do not conclude an employment contract with the applicant, the application documents will be automatically deleted six months after notification of the rejection decision, provided that no other legitimate interests on our part prevent deletion. Other legitimate interest in this sense is, for example, a duty to provide evidence in proceedings under the General Equal Treatment Act (AGG).
Applications can also reach us via recruiting platforms or external application service providers. We do not always have influence over which job postings are offered by the service providers. The respective services and service providers are responsible for compliance with data protection regulations within their sphere of influence.
We currently use the following social media plugins: LinkedIn. We use the so-called two-click solution. This means that when you visit our site, no personal data is initially passed on to the providers of the plugins. You can recognize the provider of the plugin by the mark on the box above its initial letter or logo. We open the possibility for you to communicate directly with the provider of the plugin via the button. Only if you click on the marked box and thereby activate it, the plugin provider receives the information that you have called up the corresponding website of our online offer. In addition, further data is transmitted. By activating the plugin, your personal data is transmitted to the respective plugin provider and stored there (in the case of US providers, in the USA). Since the plugin provider collects the data in particular via cookies, we recommend that you delete all cookies via the security settings of your browser.
We have neither influence on the collected data and data processing operations, nor are we aware of the full scope of data collection, the purposes of processing, the storage periods. We also have no information about the deletion of the collected data by the plugin provider.
The plugin provider stores the data collected about you as usage profiles and uses them for purposes of advertising, market research and/or demand-oriented design of its website. Such an evaluation is carried out in particular (also for users who are not logged in) for the display of tailored advertising and to inform other users of the social network about your activities on our website. You have the right to object to the creation of these user profiles, whereby you must contact the respective plugin provider to exercise this right. Via the plugins, we offer you the opportunity to interact with the social networks and other users, so that we can improve our offer and make it more interesting for you as a user. The legal basis for the use of the plugins is Art. 6 para. 1 p. 1 lit. f DSGVO.
The data transfer takes place regardless of whether you have an account with the plugin provider and are logged in there. If you are logged in to the plugin provider, the data we collect is directly assigned to your account with the plugin provider. If you click the activated button and, for example, link to the page, the plugin provider also saves this information in your user account and shares it publicly with your contacts. We recommend that you log out regularly after using a social network, but especially before activating the button, as this allows you to avoid an assignment to your profile with the plugin provider.
If, alternatively, only links to the services are included, you will be redirected to our respective page after clicking on the link, i.e. only then will data be transferred to the corresponding service.
For more information on the purpose and scope of data collection and processing by the plug-in provider, please refer to the data protection notices of these providers provided below. There you will also find further information on your rights in this regard and setting options for protecting your privacy.
Address of the plugin provider and URL with the privacy policy:
LinkedIn Corporation, 2029 Stierlin Court, Mountain View, California 94043, USA;
https://www.linkedin.com/legal/privacy-policy.
We have integrated components of the LinkedIn Corporation on this website. LinkedIn is an Internet-based social network that allows users to connect with existing business contacts and make new business contacts. Over 400 million registered individuals use LinkedIn in more than 200 countries. This makes LinkedIn currently the largest platform for business contacts and one of the most visited websites in the world.
The operating company of LinkedIn is LinkedIn Corporation, 2029 Stierlin Court Mountain View, CA 94043, USA. For data protection issues outside the USA, LinkedIn Ireland, Privacy Policy Issues, Wilton Plaza, Wilton Place, Dublin 2, Ireland, is responsible.
With each individual call-up of our website that is equipped with a LinkedIn component (LinkedIn plugin), this component causes the browser you are using to download a corresponding representation of the component from LinkedIn. Further information on LinkedIn plugins can be found at https://developer.linkedin.com/plugins. Within the scope of this technical procedure, LinkedIn receives knowledge of which specific subpage of our website is visited by you.
If you are logged in to LinkedIn at the same time, LinkedIn recognizes which specific subpage of our website you are visiting each time you call up our website and for the entire duration of your respective stay on our website. This information is collected by the LinkedIn component and assigned to your LinkedIn account by LinkedIn. If you click on a LinkedIn button integrated on our website, LinkedIn assigns this information to your personal LinkedIn user account and stores this personal data.
LinkedIn always receives information via the LinkedIn component that you have visited our website if you are logged into LinkedIn at the same time as calling up our website; this takes
place regardless of whether you click on the LinkedIn component or not. If you do not want this information to be transmitted to LinkedIn, you can prevent the transmission by logging out of your LinkedIn account before accessing our website.
LinkedIn offers the ability to unsubscribe from email messages, SMS messages, and targeted ads, as well as manage ad settings at https://www.linkedin.com/psettings/guest- controls. LinkedIn also uses partners such as Quantcast, Google Analytics, BlueKai, DoubleClick, Nielsen, Comscore, Eloqua and Lotame, which may set cookies. Such cookies can be rejected at https://www.linkedin.com/legal/cookie-policy. LinkedIn's applicable privacy policy is available at https://www.linkedin.com/legal/privacy-policy. LinkedIn's cookie policy is available at https://www.linkedin.com/legal/cookie-policy.
We use the tool "Zoom" to conduct conference calls, online meetings, video conferences and/or webinars (hereinafter: "Online Meetings"). "Zoom" is a service of Zoom Video Communications, Inc, 55 Almaden Blvd, Suite 600, San Jose, CA 95113, USA; website: https://zoom.us/.
Note: Insofar as you call up the website of "Zoom", the provider of "Zoom" is responsible for data processing. However, calling up the Internet page is only necessary for the use of "Zoom" in order to download the software for the use of "Zoom".
You can also use "Zoom" if you enter the respective meeting ID and, if necessary, further access data for the meeting directly in the "Zoom" app. If you do not want to or cannot use the "Zoom" app, the basic functions can also be used via a browser version, which you can also find on the "Zoom" website.
Various types of data are processed when using "Zoom". The scope of the data also depends on the data you provide before or during participation in an "online meeting".
The following personal data are subject to processing:
survey functions in an "online meeting". To this extent, the text entries you make are processed in order to display them in the "online meeting" and, if necessary, to log them. To enable the display of video and the playback of audio, the data from the microphone of your terminal device and from any video camera of the terminal device will be processed accordingly for the duration of the meeting. You can switch off or mute the camera or microphone yourself at any time via the "Zoom" applications.
To participate in an "online meeting" or to enter the "meeting room", you must at least provide information about your name.
We use "Zoom" to conduct "online meetings". If we want to record "online meetings", we will transparently communicate this to you in advance and - if necessary - ask for consent. The fact of the recording will also be displayed to you in the "Zoom" app.
If it is necessary for the purposes of logging the results of an online meeting, we will log the chat content. However, this will usually not be the case.
In the case of webinars, we may also process questions asked by webinar participants for the purposes of recording and following up webinars.
If you are registered as a user at "Zoom", then reports on "online meetings" (meeting metadata, telephone dial-in data, questions and answers in webinars, survey function in webinars) can be stored at "Zoom" for up to one month.
The option of software-based "attention monitoring" ("attention tracking") that exists in "Online Meeting" tools such as "Zoom" is deactivated.
Legal bases of data processing:
Insofar as personal data of our employees is processed, Section 26 BDSG is the legal basis for data processing. If, in connection with the use of "Zoom", personal data is not required for the establishment, implementation or termination of the employment relationship, but is nevertheless an elementary component in the use of "Zoom", Art. 6 (1) lit. f DSGVO is the legal basis for data processing. In these cases, our interest is in the effective implementation of "online meetings".
Incidentally, the legal basis for data processing when conducting "online meetings" is Art. Art. 6 Abs. 1 lit. b DSGVO, insofar as the meetings are conducted in the context of contractual relationships.
If there is no contractual relationship, the legal basis is Art. 6 (1) lit. f DSGVO. Here, too, our interest is in the effective implementation of "online meetings".
Recipients; Disclosure of Data:
Personal data processed in connection with participation in "online meetings" will not be disclosed to third parties as a matter of principle unless it is specifically intended for disclosure. Please note that the content of "online meetings", as well as personal meetings, is often used to communicate information with customers, interested parties or third parties and is therefore intended to be passed on. The provider of "Zoom" necessarily obtains knowledge of the above-mentioned data to the extent that this is provided for in the context of our order processing agreement with "Zoom".
We have concluded an order processing agreement with the provider of "Zoom" that complies with the requirements of Art. 28 DSGVO.
An adequate level of data protection guaranteed by the conclusion of the so-called EU standard contractual clauses.
Zoom's privacy policy can be found at https://zoom.us/docs/de-de/privacy-and-legal.html.
In the following, we share the legal basis of the General Data Protection Regulation (GDPR) on the basis of which we process personal data. Please note that in addition to the regulations of the GDPR, the national data protection regulations in your or our country of residence and domicile may apply.
In addition to the data protection regulations of the General Data Protection Regulation, national regulations on data protection apply in Germany. These include, in particular, the Act on Protection against Misuse of Personal Data in Data Processing (Federal Data Protection Act - BDSG). In particular, the BDSG contains special regulations on the right to information, the right to erasure, the right to object, the processing of special categories of personal data, processing for other purposes and transmission, as well as automated decision-making in individual cases, including profiling. Furthermore, it regulates data processing for employment purposes (Section 26 BDSG), in particular with regard to the establishment, implementation or termination of employment relationships as well as the consent of employees. Furthermore, state data protection laws of the individual federal states may apply.
Art. 6 Abs.1 lit. a DSGVO serves our company as the legal basis for processing operations in which we obtain consent for a specific processing purpose.
If the processing of personal data is necessary for the performance of a contract to which the data subject is a party, as is the case, for example, with processing operations that are necessary for a delivery of goods or the provision of another service or consideration, the processing is based on Article 6 (1) lit. b DSGVO. The same applies to such processing operations that are necessary for the implementation of pre-contractual measures, for example in cases of inquiries about our products or services.
If our company is subject to a legal obligation by which the processing of personal data becomes necessary, such as for the fulfillment of tax obligations, the processing is based on Art. 6 para. 1 lit. c DSGVO.
Ultimately, processing operations may be based on Art. 6 (1) lit. f DSGVO. Processing operations that are not covered by any of the aforementioned legal bases are based on this legal basis if the processing is necessary for the protection of a legitimate interest of our company or a third party, provided that the interests, fundamental rights and freedoms of the data subject are not overridden. Such processing operations are permitted to us in particular because they were specifically mentioned by the European legislator. In this respect, it took the view that a legitimate interest could be assumed if the data subject is a customer of the controller (recital 47 sentence 2 DSGVO).
If the processing of personal data is based on Article 6 I lit. f DSGVO, our legitimate interest is the efficient performance of our business activities for the benefit of the well-being of our employees and our shareholders.
We process and store your personal data only for the period required to fulfill the purpose of storage or if this has been provided for in laws or regulations. After discontinuation or fulfillment of the purpose, your personal data will be deleted or blocked. In the case of blocking, deletion will take place as soon as legal, statutory or contractual retention periods do not conflict with this and there is no reason to assume that deletion would impair your interests worthy of protection, as well as deletion would not cause a disproportionately high expense due to the special nature of the storage.
Otherwise, specific retention period criteria are provided in the individual sections of this Privacy Notice.
You have the possibility at any time to review, modify or delete the personal data provided to us by sending us an email to This email address is being protected from spambots. You need JavaScript enabled to view it.In this way, you can also opt out of receiving further information in the future.
Likewise, you have the right to revoke once granted consent with effect for the future at any time.
The data processed by us will be deleted in accordance with the legal requirements as soon as their consents granted for processing are revoked or other permissions cease to apply (e.g. if the purpose of processing this data has ceased to apply or it is no longer required for the purpose).
If the data is not deleted because it is required for other and legally permissible purposes, its processing is restricted to these purposes, i.e. the data is blocked and not processed for other purposes. This applies, for example, to data that must be retained for reasons of commercial or tax law or whose storage is necessary for the assertion, exercise or defense of legal claims or for the protection of the rights of another natural or legal person.
We would like to inform you that the provision of personal data is partly required by law (e.g. tax regulations) or may also result from contractual regulations (e.g. information on the contractual partner). Sometimes, in order to conclude a contract, it may be necessary for a data subject to provide us with personal data that must subsequently be processed by us.
For example, the data subject is obliged to provide us with personal data if our company concludes a contract with him or her. Failure to provide the personal data would mean that the contract with the data subject could not be concluded.
Before the data subject provides personal data, the data subject must contact one of our employees. Our employee will inform the data subject on a case-by-case basis whether the provision of the personal data is required by law or by contract or is necessary for the conclusion of the contract, whether there is an obligation to provide the personal data, and what the consequences of not providing the personal data would be.
As a responsible company, we do not use automated decision-making.
Changes in the law or changes to our internal processes may make it necessary to adapt this data protection notice. We ask you to inform yourself regularly about the content of our data protection information.
Please note that the current version of the privacy policy is the valid one. Status: 18.01.2021
Note: This data protection notice was prepared using a wide range of sources, including the links provided here. Current case law as well as interpretations and commentaries have been taken into account as far as we are aware.